Eurobio Scientific
EurobioPlex
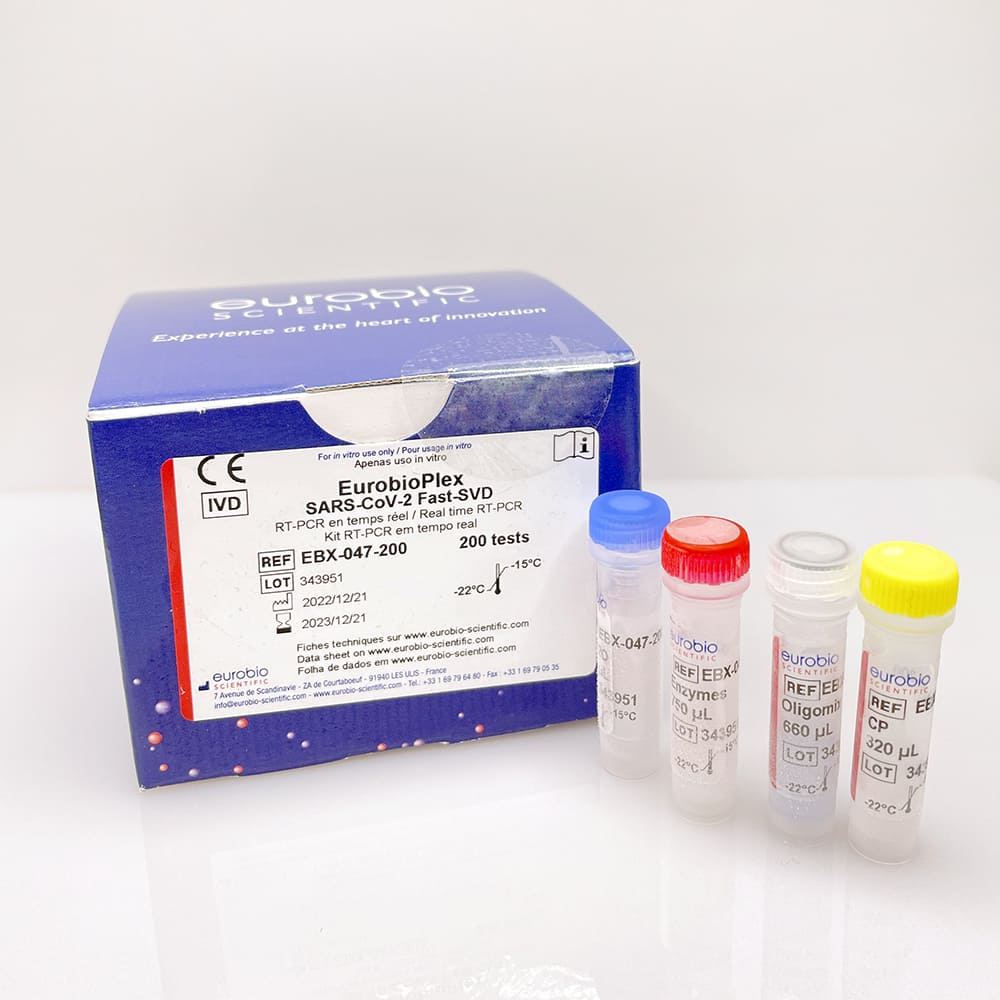
EurobioPlex SARS-CoV-2 Fast-SVD Screening and Variants Detection
EBX-047
The EurobioPlex SARS-CoV-2 Fast-SVD test is a real-time polymerase chain reaction (RT-PCR) assay which, in a single test, identifies the prevalence of SARS-CoV-2 and distinguishes be-tween the K417N (Omicron and Beta variant screening), E484K (Beta and Gamma variant screening) and L452R (Delta variant screening) mutations in a qualitative manner. The pres-ence of the K417N mutation alone (without the E484K mutation) allows the Omicron variant to be differentiated from the Beta variant and to screen for the Omicron variant. If the K417N mutation is detected, then the sample should be studied in sequencing in order to screen for the Omicron variant (and thus differentiate it from the beta variant).
- SARS-CoV-2/ K417N / L452R / E484K / Housekeeping gene
- CE-IVD
- Packaging:50, 100, 200, 600 tests
- Qualitative
- Nasopharyngeal swab
You have questions or want more informations?
- REF : EBX-047
- Pathogens : SARS-CoV-2/ K417N / L452R / E484K / Housekeeping gene
- Shipment : Dry ice
- Storage at -20 °C
- EurobioPlex Thermocycleur : CFX96™Real Time PCR detection system (Bio-Rad)
- EurobioPlex Extractor : RNA extraction Suitable for Respiratory specimens, Maelstrom 9600 TanBEAD, OptiPure Viral Auto Plate
| SARS CoV-2 | K417N Mutation | L452R Mutation | E484K Mutation | |
| Analytical Sensitivity | 5 copies / µl | 5 copies / µl | 5 copies / µl | 5 copies / µl |
| Diagnostic sensitivity | > 99% | > 99% | > 99% | > 99% |
| Diagnostic specificity | > 99% | > 99% | > 99% | > 99% |
Not a member yet? Register now
You may also like
Related products
-
Bacteriology
EurobioPlex Strepto B
-
Bacteriology
EurobioPlex Bartonella
-
Bacteriology
EurobioPlex Nosocomial bacterial meningitis
-
Bacteriology
EurobioPlex Community-acquired bacterial meningitis
Our Products & Services
For the field of transplantation, the group produces reagents and software for transplant matching and chimerism monitoring and culture media for cornea graft transplants.
Eurobio Scientific manufactures prognostic genetics tests for breast cancer and prostatic cancer.
The group develops and markets genetic molecular tests to detect a wide variety of illnesses, including detecting infectious diseases, fertility testing or assaying dermatological conditions.
To support the research and development field, Eurobio Scientific has a broad portfolio in Life Sciences products including cell culture media and quality controls.